
Test development is a Roche and Lilly collaboration to support earlier Alzheimer’s diagnosis.
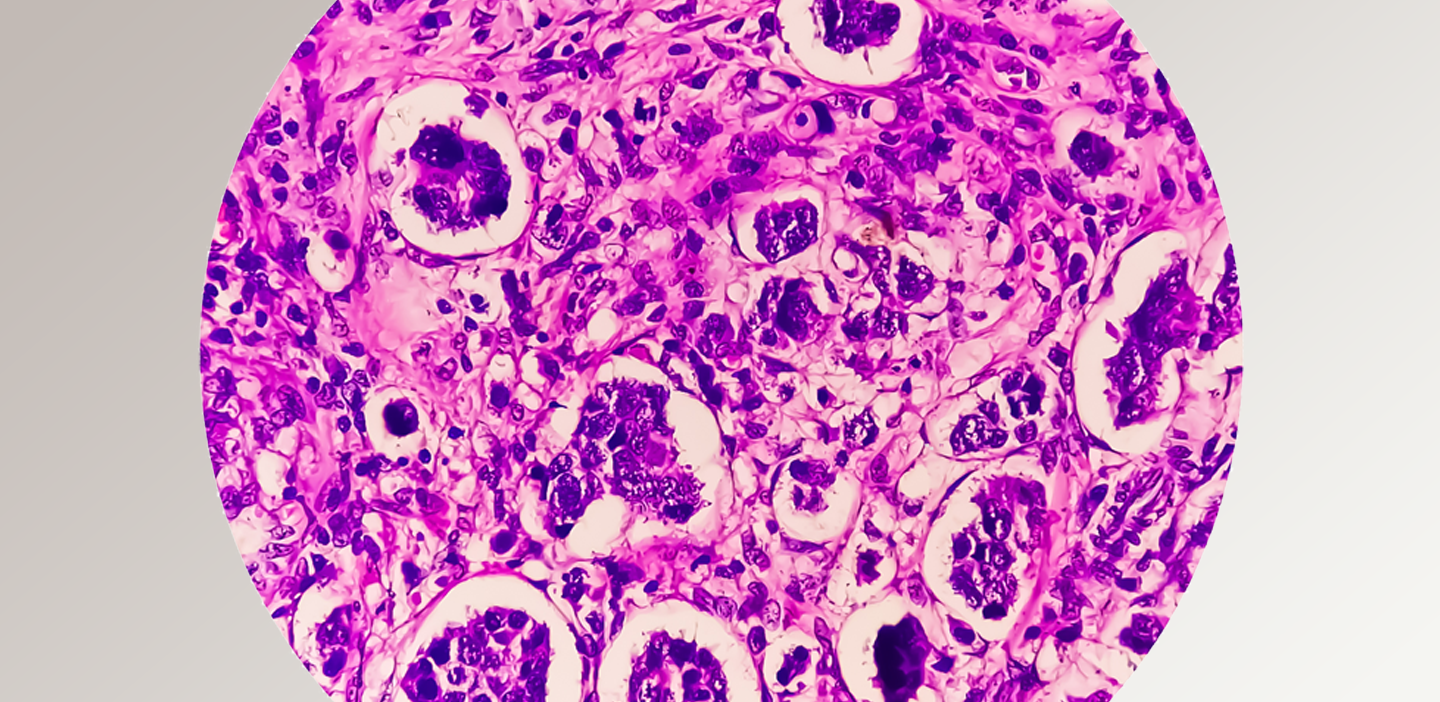
One of our experts answers questions about innovations in tissue and digital pathology.

In a world where you can be anything, be a laboratory professional.

Thought leaders and cancer experts drive advancements in pathology and oncology to improve outcomes and shape the future.
Learn more about our portfolio of lab instruments, systems, assays and more

More than 800,000 documents available on demand — always up-to-date with 24/7 access