
Roche and GenMark are dedicated to providing world-class solutions for multiplex testing.

Test development is a Roche and Lilly collaboration to support earlier Alzheimer’s diagnosis.
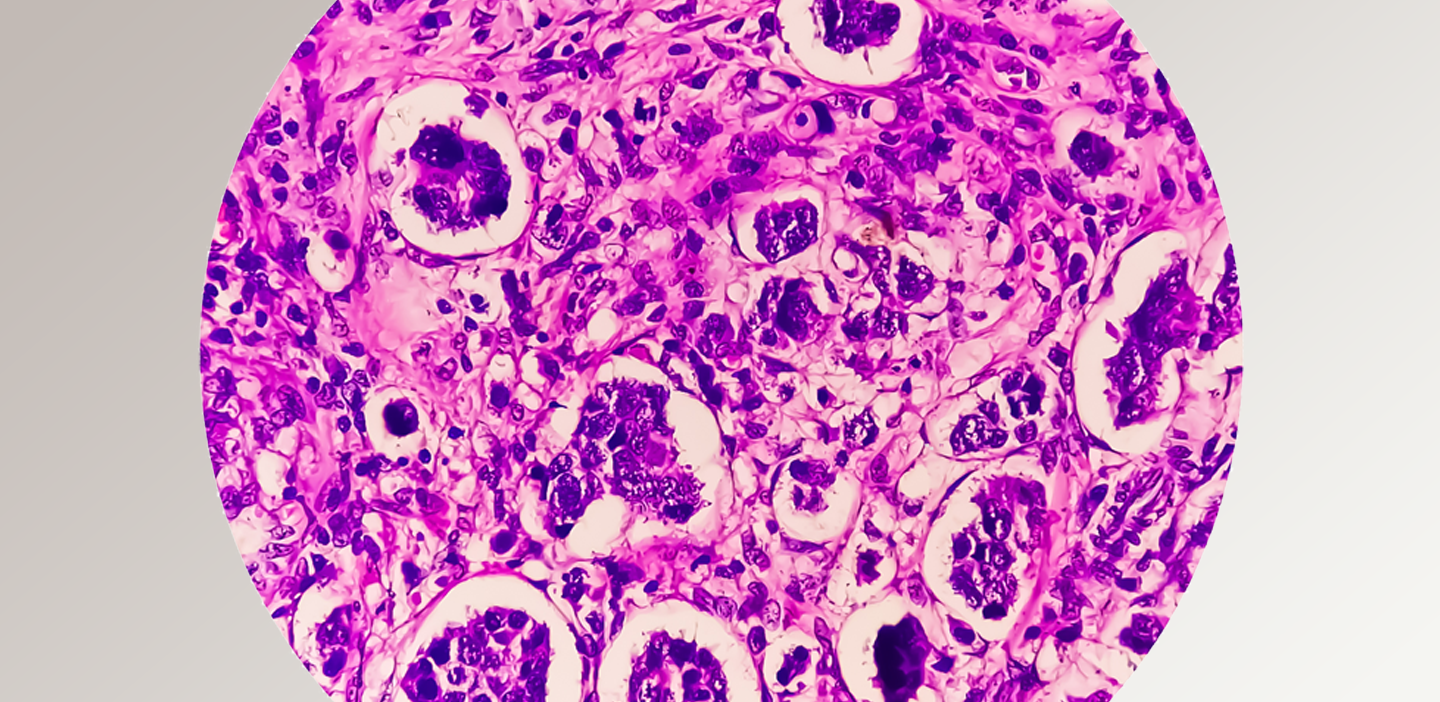
One of our experts answers questions about innovations in tissue and digital pathology.

Join Roche at Booth No. 4, May 17-18, 2024 at PASCV 2024, in Clearwater Beach, FL.
Learn more about our portfolio of lab instruments, systems, assays and more

More than 800,000 documents available on demand — always up-to-date with 24/7 access